- Imaging studies for the initial evaluation of Hodgkin lymphoma should include a screening chest radiograph and computed tomography (CT) of the chest, abdomen, and pelvis (with intravenous contrast if possible). CT is the gold-standard imaging modality. Positron emission tomography (PET) is an important adjunct to CT for initial staging.
- An improved histopathologic classification, accurate staging, improved radiotherapy, and effective chemotherapeutic agents contribute to the high cure rate for Hodgkin lymphoma. For therapeutic success, care should be given by a multidisciplinary team with expertise in histopathology, diagnostic radiology, medical oncology, and radiation therapy.
- Because of the high cure rate of Hodgkin lymphoma, long-term toxicity of therapy is becoming increasingly important as the follow-up time for patients increases. The full toxicity profile of radiation is only now becoming apparent. The risk of several types of solid tumors is dramatically increased in patients who receive radiation therapy for Hodgkin lymphoma.
Latest Updates
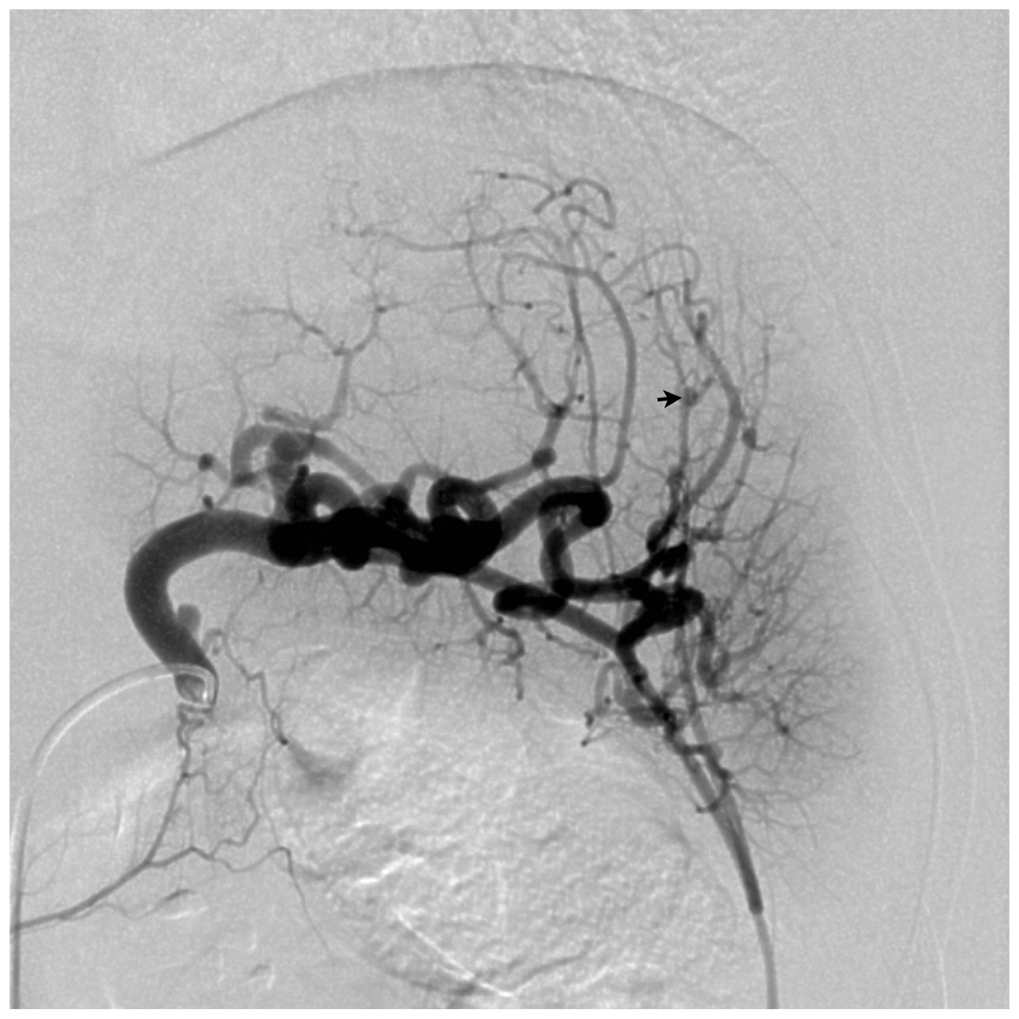
- Visceral artery aneurysms are increasingly identified with the widespread use of advanced imaging techniques.
- Increasing incidence is thought to be linked with the rise in percutaneous biliary procedures, endovascular chemoembolization therapies, liver transplantation, arterial trauma secondary to laparoscopic manipulation of vessels, and a trend toward nonoperative management of blunt liver trauma.
- Management is evolving and includes open repair, laparoscopic and robotic-assisted repair, and a more important role for endovascular therapies.
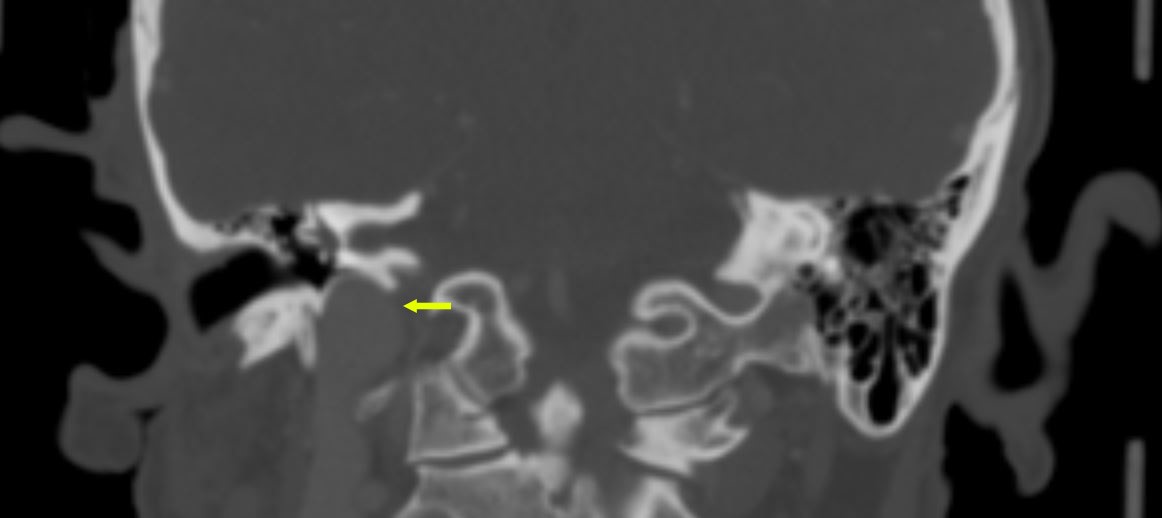
- Immunotherapy for H&N cancer provides alternative treatment option for patients with certain TB cancers.
- Various biomarkers continue to be investigated for correlation of prognosis and surveillance.
- Multidisciplinary, multi-institutional collaborative research is needed to determine optimal treatment for TB cancers.
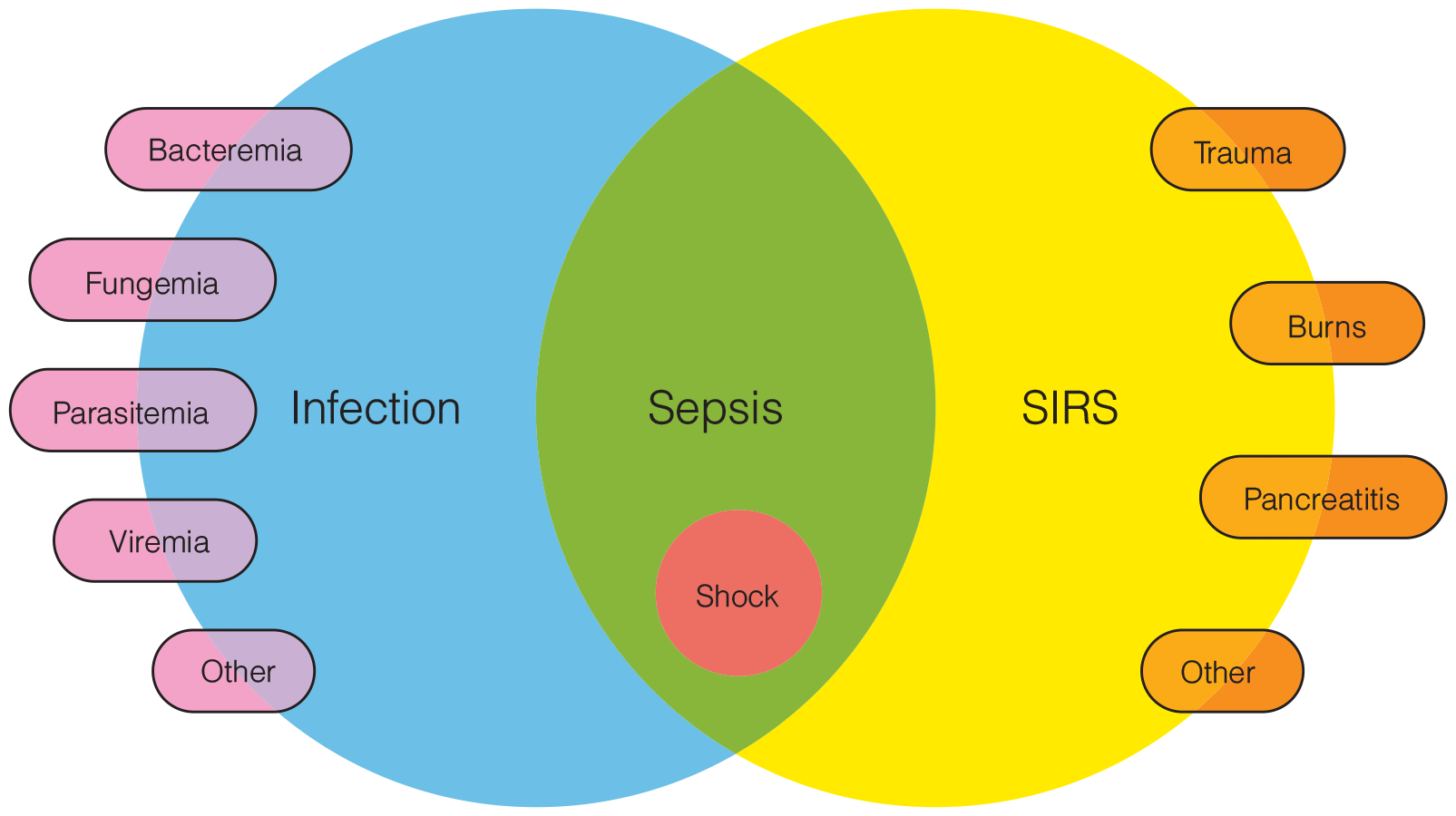
Prevention and Diagnosis of Infection
- Microbiologic studies are critical for characterizing infections. Gram stains and cultures of wound tissue, pus, sputum, urine, and drainage effluent are generally very useful. Identification of not only the particular organism involved but also of its specific antimicrobial susceptibility has become common practice in most hospital clinical laboratories.
- Treatment of CAUTI requires removal or change of the catheter along with systemic antimicrobial therapy. The predominant microorganisms causing CAUTI in the ICU are enteric gram-negative bacilli, Candida species, enterococci, staphylococci, and Pseudomonas aeruginosa. Multidrug resistance is a significant problem in urinary pathogens

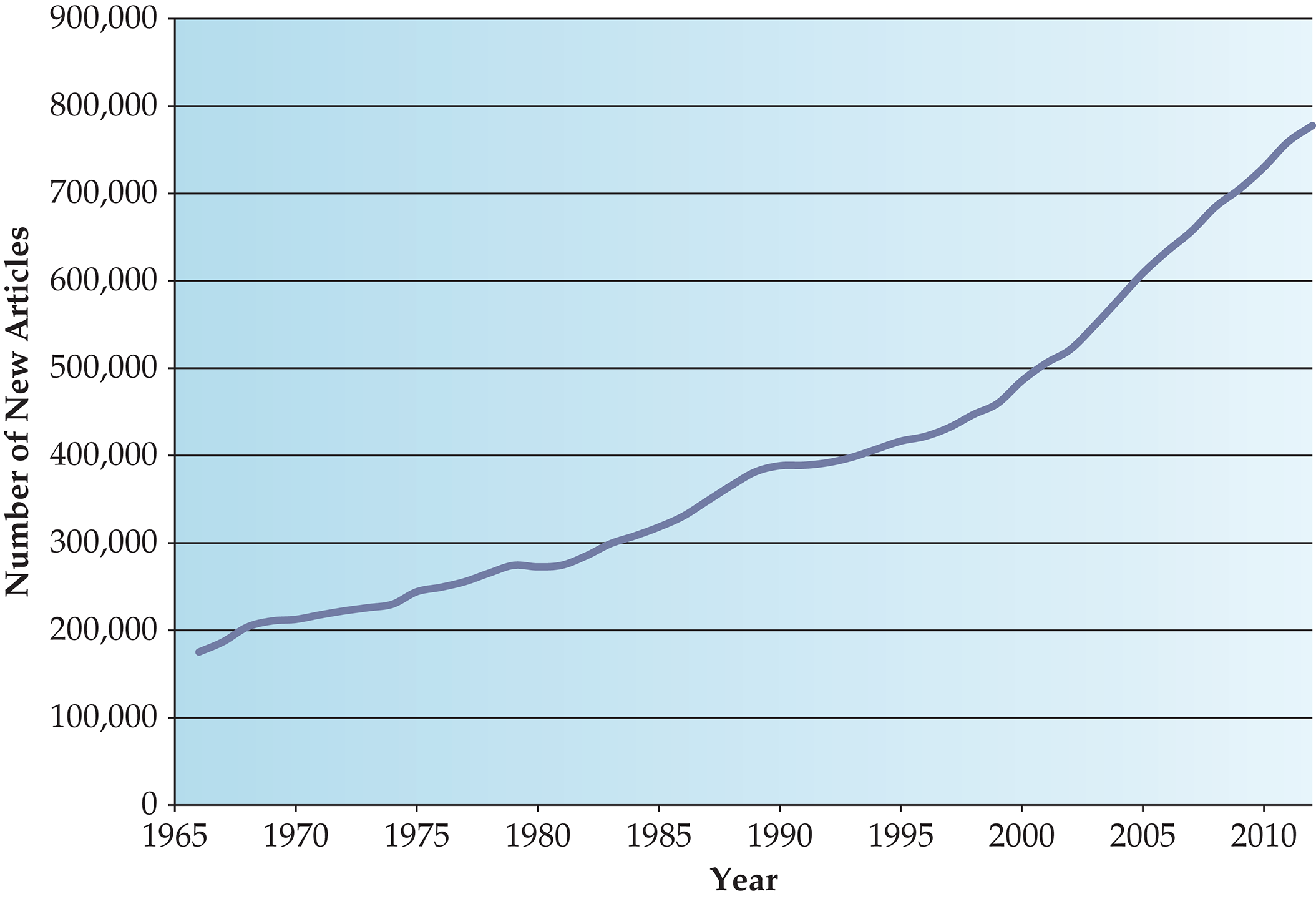
Practicing Evidence-Based Medicine
- Even the most competent physician can be prone to misusing epidemiologic concepts. An example of inaccurate decision making, resting on Bayes’s theorem, occurred in a recent study in which primary care physicians were given clinical scenarios. Although the clinicians confidently provided their estimates of the probabilities of given disorders, no consensus could be found among the estimates. Another study assessed the ability of medical students, residents, and attending physicians to correctly determine the positive predictive value of a hypothetical screening test. The vast majority of respondents not only got the question wrong but also had an answer that would have led to the opposite clinical conclusions, guessing an incorrect positive predictive value of 95% when the true answer was 2%.
- Clinical gestalt can also accurately assess the pretest probability of PE.
- For patients with a low pretest probability of PE, the Pulmonary Embolism Rule-out Criteria can be used to rule out PE without further testing including no need to order a
- D-dimer.
- The YEARS protocol may be used to exclude a subset of patients from having a workup to rule out PE when their D-dimer is less than 1000 as opposed to 500.
- Novel or new oral anticoagulants are becoming the mainstay of treatment for the hemodynamically stable patient with PE.
- Intravenous alteplase, catheter-directed thrombolysis, surgical embolectomy, and catheter-directed embolectomy are treatment modalities for patients with PE who are hemodynamically unstable.
- For patients in imminent or actual PE-related cardiac arrest, current guidelines recommend a bolus regimen consisting of 50 mg IV t-PA given over two minutes and repeated after 15 minutes in the absence of return of spontaneous circulation.
- A subset of patients with PE can be treated as outpatients if their Simplified Pulmonary Severity Index is 0.
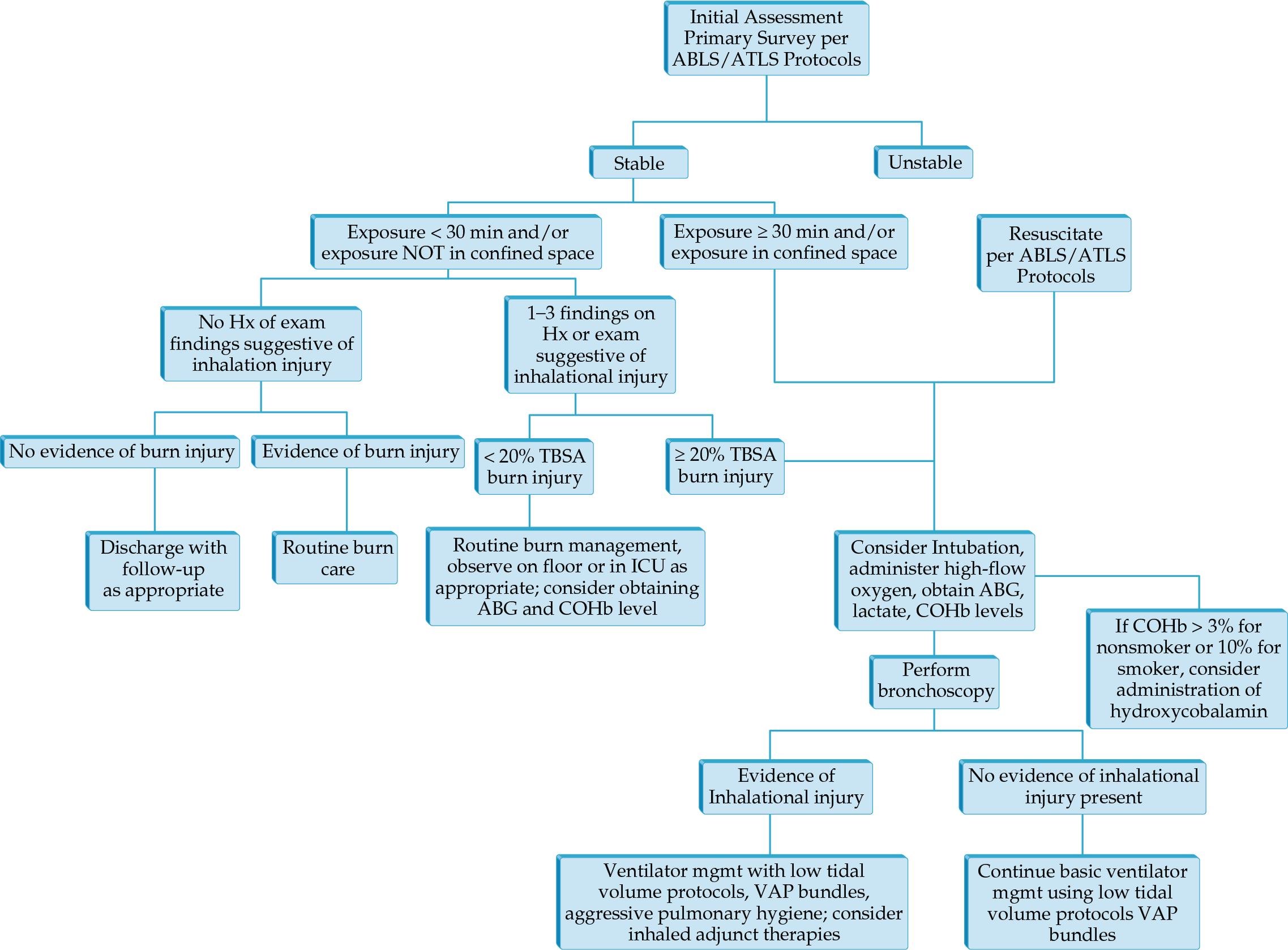
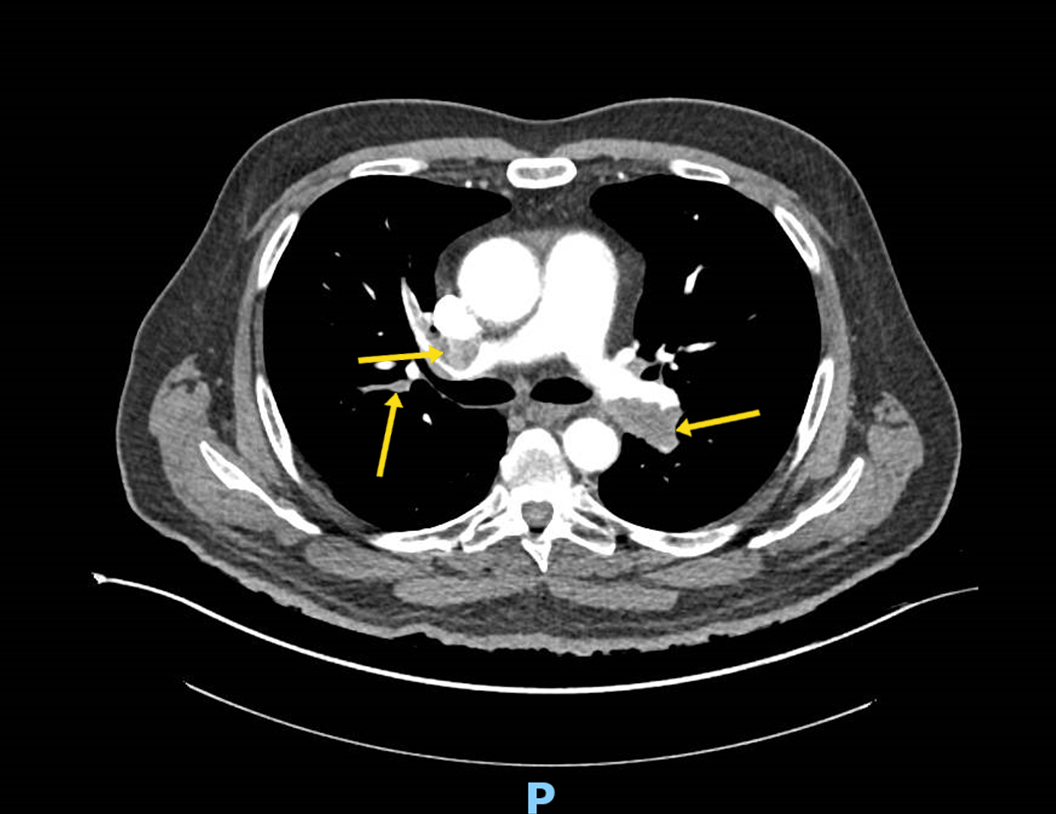
- CT of the chest has taken a greater role in determining injury severity in inhalation injury.
- Airway control and ventilator management remain the mainstays of treatment in those with severe injury.
- Volumetric diffusive respiration is a mode specifically developed for inhalation injury that has been shown to decrease use of other rescue modes of ventilation.
Hematology: Chronic Lymphocyte Leukemia and Other Chronic Lymphoid Leukemias
Lymphocyte immunophenotyping by flow cytometry can distinguish between malignant (clonal) and nonmalignant (nonclonal) causes of lymphocytosis and eliminates the need to rely on the duration or magnitude of the lymphocyte count elevation to differentiate CLL and other lymphoproliferative disorders from reactive causes of lymphocytosis.
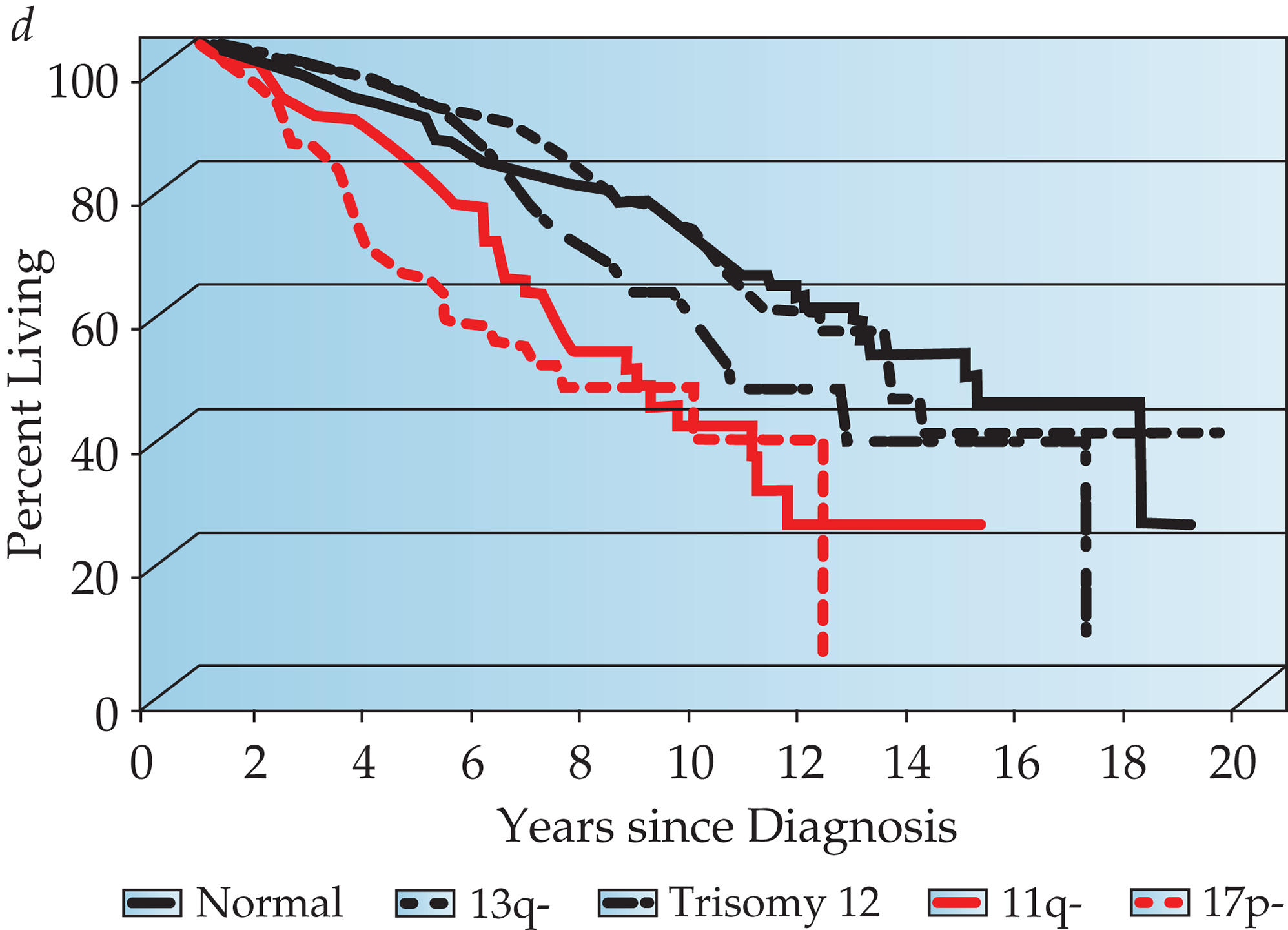
Chromosome analysis by FISH predicts patient survival. In a retrospective analysis of a heterogeneous patient population, many of whom had advanced-stage disease and were previously treated, Dohner and colleagues developed a hierarchical system that assigns patients to one of five categories with widely different median survival.
The development and therapeutic application of anti-CD20 monoclonal antibodies in the 1990s revolutionized the care of patients with lymphoid malignancy. Several trials evaluated the efficacy of combining monoclonal antibodies with chemotherapy (CIT) for patients with CLL.


.png)






